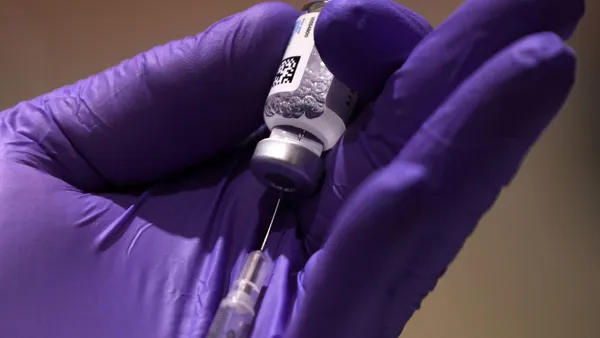
What’s on your Mind Opinions Monitoring Drug Safety The industry and the U.S. Food and Drug Administration have come under significant scrutiny since Merck’s withdrawal of Vioxx. Six papers on the issue were published on the subject in the Dec. 1, 2004, issue of the Journal of the American Medical Association. The journal’s editors called for a new, independent office separate from the FDA to monitor drugs after they’re on the market. Merck and the FDA have been accused of moving too slowly to stop sales of the arthritis drug Vioxx, which Merck withdrew in September after revealing it raised the risk of heart attacks and strokes. Some scientists claim that painkillers similar to Vioxx, especially Pfizer Inc.’s Bextra, also carry risks. Dr. David Graham, associate director of science in the FDA’s Office of Drug Safety, told a Senate panel that the FDA was incapable of protecting the public and that at least five other drugs are on the market that should be looked at seriously to see whether they should remain there. He cited the acne drug Accutane, the weight-loss drug Meridia, the anticholesterol drug Crestor, the pain reliever Bextra, and the asthma drug Serevent. PharmaVOICE asked: Are companies and regulators doing enough to protect consumers? What needs to be done to ensure that drugs are safe once on the market? And is a new regulatory body needed to oversee drug safety? FDA improvements in drug safety monitoring Editor’s Note: As this issue went to press, HHS Secretary Mike Leavitt and FDA Commissioner Lester M. Crawford unveiled a new emboldened vision for FDA that will promote a culture of openness and enhanced oversight within the agency. As part of this vision, the FDA will create a new independent Drug Safety Oversight Board (DSB) to oversee the management of drug safety issues, and it will provide emerging information to health providers and patients about the risks and benefits of medicines. FDA Commissioner Crawford announced specific proposals for immediate and fundamental steps to improve the way the FDA manages drug safety information. These proposals focus on making the FDA’s review and decision-making processes more independent and transparent. The DSB will oversee the management of important drug-safety issues within the Center for Drug Evaluation and Research (CDER). The DSB will comprise members from the FDA and medical experts from other HHS agencies and government departments (i.e., Department of Veterans Affairs) who will be appointed by the FDA commissioner. The board also will consult with other medical experts and representatives of patient and consumer groups. The FDA also will increase the transparency of the agency’s decision-making process by establishing new, and expanding existing, communication channels to provide targeted drug-safety information to the public. These channels will be used to help ensure that established and emerging drug safety data are quickly available in an easily accessible form. The increased openness will enable patients and their healthcare professionals to make better-informed decisions about individual treatment options. The agency is proposing a new “Drug Watch” Web page for emerging data and risk information and increased use of consumer-friendly information sheets written especially for healthcare professionals and patients. As the FDA develops these communications formats, the agency will be soliciting public input on how regulatory officials should manage potential concerns associated with disseminating emerging information before regulatory action. The agency will issue draft guidance on procedures and criteria for identifying drugs and information for the Drug Watch Web page. In addition, the FDA will actively seek feedback from healthcare professionals and patients on how best to make this information available to them. A cornerstone of all information collection, evaluation, and communication proposals in an age of increasing electronic health information must be a strict adherence to maintaining patient privacy. The FDA is committed to maintaining patient privacy as it undertakes these steps. Separately, a panel of advisers to the FDA has recommended keeping Celebrex, Vioxx, and Bextra on the market but with restrictions, including black-box warnings and a ban on direct-to-consumer advertising. Safety from a small- company perspective Because of recent safety problems with marketed drugs, especially with new chemical entity (NCE) drugs approved within the past five years, the FDA has been pressured to make changes in its processes. These suggested changes include fixing the FDA review system, overhauling the FDA, investigating the drug developers, and setting up an independent Safety Review Board. At this time, Cornerstone BioPharma, a Research Triangle Park, N.C.-based specialty pharmaceutical company currently focused on the development and commercialization of niche prescription medications in the pain, anti-infective, and respiratory markets, is not developing any NCE drugs and like the FDA, views safety as an essential area of the drug-development process that must be fully assessed before putting drugs into clinical trials, and monitored once the drugs are approved. I equate the development of any new drug to driving down a four-lane highway at the maximum speed allowed. One lane is occupied by the consumers, the second lane is occupied by the medical community, the third lane has regulatory agencies, and the fourth lane — the fast lane — is super loaded by the manufacturers/researchers who are vying to be first to market with a new drug with greater efficacy. All of these lanes are headed to a single lane ahead — the commercial approval road. At this point in time, because of questions on the amount of safety data needed on NCEs and recently approved NCEs, the authorities are asking the developers to leave the highway and to take a detour while safety is being assessed. Meanwhile, the consumers/patient advocates are driving down the lane expecting to be treated with the newest and most effective drugs, and healthcare prescribers are in gear looking for the best products to prescribe to their patients. The drug developers are driving down the fast lane seeking the fastest approval possible on drugs that fill a specified medical need. The FDA/regulators have taken this detour, yet are still driving very cautiously while dealing with the expectations of the patients, healthcare professionals, and the drug developers. How long the detour lasts is yet to be determined. If, during investigations of the particular drugs, it is found that not all of the data were revealed on a timely basis, more safety data should be gathered via preclinical studies; or larger clinical trials and/or longer trials are needed, this does not automatically imply the need for a Safety Review Board independent of the FDA. It does show us that the internal review procedures at developers and the FDA, the FDA development guidelines, and perhaps, the safety requirements of the FDA regulations need revision. One thing that is certain: industry and the regulators fully support reducing the unforeseen risks after drugs are approved. But how to reduce the risks is a road less traveled until more is learned after investigating the specific drugs in question. Larry Tamura Director Regulatory/Quality Assurance Cornerstone BioPharma Inc. 100 people, 100 responses I have no objection to the FDA monitoring drug safety. Although I also can see a great use in having a separate group (and additional devoted people) focusing upon the drug safety and risk management of pharmaceutical products, the FDA knows the drug the best from early clinical trials through post-marketing. My main concern is that even an independent group would still be subject to criticism. No drug is without risks. The issue of drug safety is really a balance of risks and benefits in a variety of patient populations across a wide range of drugs, thus leaving wide open the interpretation as to what is an acceptable benefit-risk ratio. It is very likely that if you asked 100 people, you would get 100 different responses. There needs to be one group that makes this decision for the nation as a whole, and it should err on the side of conservatism when effective drugs already exist. Matthew W. Reynolds, Ph.D. Senior Director, Risk Management and Safety Services, MetaWorks Inc. Restoring confidence The pharma industry already is heavily regulated. But there obviously are serious questions now being raised within the public about the effectiveness of those regulations. An independent review for marketed products would help re-establish some of that confidence. Perhaps that same agency could be charged with the responsibility of providing and analyzing summaries of published data that might help the public and prescribing doctors make the best choices for drug prescribing. There appears to be a tendency to prescribe the most heavily promoted drugs and the budget for promotion seems to be heavily weighted by patent-protection considerations, rather than best science. Old drugs (no patent protection) many times are still the best therapeutic choice, but their sales don’t generate enough profit to justify the promotional effort to combat the “new” drug being introduced. The long-time safety record of the older drugs may actually have less risk. Donald P. Verbarg Director Technology Development Fluid Air Inc. Taking responsibility as an industry The answer does not lie in establishing another regulatory agency; the one we have needs to do a better job with the responsibilities it already has. I also believe that we, on the industry side, need to constantly remember that we are privileged to work in a very special industry that makes a significant contribution to bettering the overall human condition. With that comes a considerable societal responsibility to do all in our power to help assure that we do no harm. As members of corporations, we may at times seem to be caught between conflicting masters with our responsibility to society and to our shareholders. But in reality, not serving the former will eventually prove deleterious to the latter. The solution resides in the establishment we already have and within each and everyone of us privileged to work in this great industry. Bill Quinlivan Marketing Partner AD-TECH Communications Stricter adherence to the IND process In my view, the last thing that we need in today’s regulatory environment is another committee having oversight in a small, highly specialized area such as drug safety. When Celebrex and Vioxx were developed, approved, and launched, their main differentiator was a safer profile in GI bleed. They were developed because there was a need, and patients were at risk for NSAID side effects that precipitated as many as 15,000 deaths a year in the United States. So, the drugs were approved for short-term use. In parallel, researchers were looking at the activity of COX-2s and recognized that effective angiogenesis might have beneficial effects in potentially preventing colon polyps from developing into colon cancer. It’s here that someone didn’t connect the dots, or if they did, they didn’t communicate the results. If a drug restricts blood flow in the colon, one had to ascertain that in long-term use that restriction, which is not specific to colon tissue, would also have a deleterious effect in the heart. The data to assess the risk were resident both at the FDA and at Merck, and it should have been reviewed when an IND was established to study the drug in these new indications. We have an IND process. I think the critical step is to enforce this process more rigidly and have greater transparency on why clinical studies are being done under that process and the basic science behind those decisions. Jim Clifford Group Company Chairman CommonHealth Pardon us … In the February 2005 article, The PC approach to improving the detail, the Website listed for INFLUENT, Pharmedica’s Tablet PC Professional Detailing division, was incorrect. To learn more about INFLUENT and its services, please visit influent.cc. PharmaVOICE apologies for the error. What’s Your Opinion? leadership development In demanding times, leadership is a competitive edge. Good leaders provide opportunities for others to grow their communication, decision-making, problem-solving, and other related skills. Leading is setting direction and guiding others to follow that direction. Good leaders debate, clarify, and enunciate values and beliefs; fuel, inspire, and guard the shared vision; ask big picture questions and “what ifs”, as well as encourage thinking the unthinkable. But leadership development is an effort, hopefully, planned in nature, that enhances the capacity to lead people. PharmaVOICE wants to know how does your company develop leaders? What’s your opinion? Please e-mail your comments to [email protected].
An article from


What's on Your Mind -- Opinions
Filed Under:
Policy & Regulation