The name “Pfizer” became synonymous during the height of the pandemic with the COVID-19 vaccine the company developed along with its partner BioNTech. Very rarely does a pharma company reach household name status, but the drugmaker became one of only a couple public faces of the industry’s response to the health crisis.
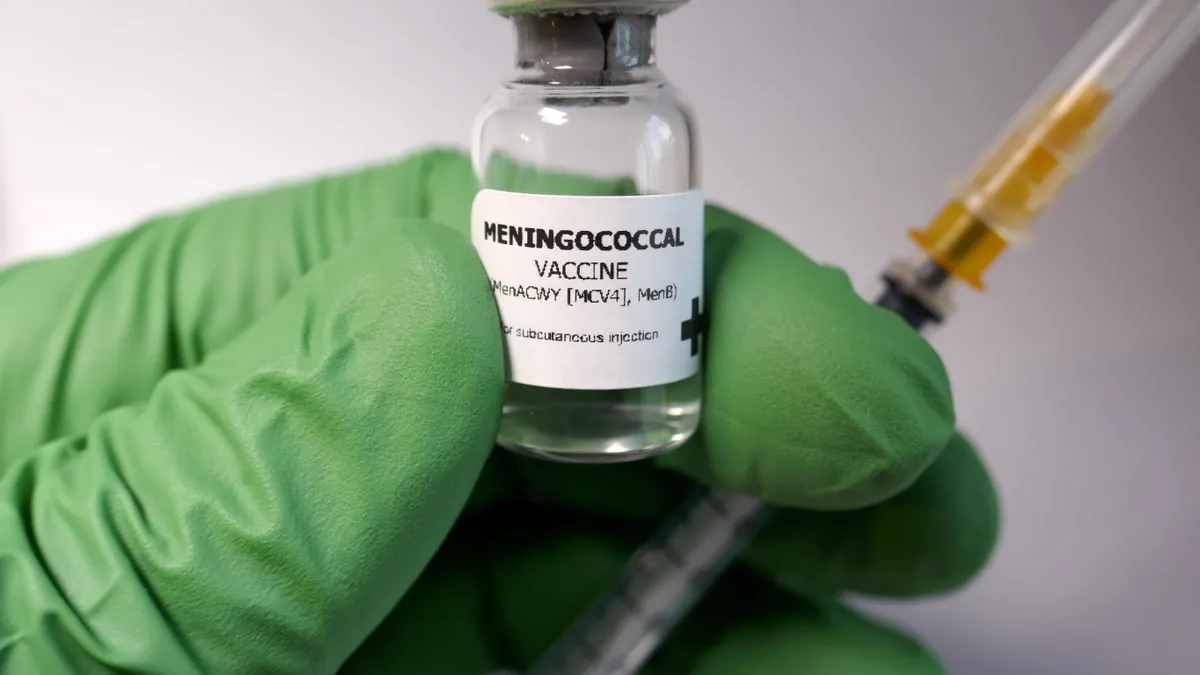
On Friday, Pfizer notched another win in their growing vaccine portfolio with an approval that flew a little further under the radar: The FDA approved Pfizer’s Penbraya, which is the first vaccine to prevent against the five most common types of meningococcal disease in adolescents.
The go-ahead marks a step in growing Pfizer’s immunization business that became supercharged by the pandemic and prepared the company to become one of the leading vaccine makers in Big Pharma.
Penbraya combines immunizations for meningococcal groups A, B, C, W and Y, which allows people aged 10 to 25 to be inoculated to all five infection types with only one shot and a follow up, a strategy that Pfizer is familiar with from years developing multivalent vaccines like its Prevnar 20 pneumococcal shot. Penbraya covers the components from two of Pfizer’s previous meningococcal vaccines — Trumenba and Nimenrix, although the latter is not licensed in the U.S.
The company said in a release that the vaccine for meningococcal disease — which can cause life-long disability or in some cases be fatal — would streamline the standard of care and potentially lead to greater vaccination rates. About nine in 10 young people are not adequately protected, according to an expert cited by Pfizer.
Here’s a look at what Pfizer has accomplished in the vaccine space and what’s currently in the pharma giant’s immunization pipeline.
The Pfizer vaccine portfolio
Back in 2009, Pfizer merged with the pharma company Wyeth and in the process gained its first vaccine called Prevnar 13, a pneumococcal conjugate vaccine that protects against infections that can cause pneumonia. Pfizer continued to develop the Prevnar group to contain seven more types of the pneumococcal types, and Prevnar 20 was approved in 2021.
Last year, the Prevnar group of vaccines brought in more than $6.3 billion in worldwide sales for Pfizer, which was a 20% jump over the year before. And this April, the shot was approved for use in children under 17, widening the market to compete with other pneumococcal vaccines like Vaxneuvance from Merck & Co. and Affinivax from GSK.
And then there’s Comirnaty. Pfizer’s COVID-19 story has become legendary and called by some “one of the greatest accomplishments in modern medicine.” After Pfizer and BioNTech brought about the first authorized (and then fully approved) vaccine for COVID-19, the company has been working to update the shot for current strains of the virus.
Although the company has had to cut jobs to save money while the sales volume of Comirnaty shots dwindle, the FDA cleared vaccines for the 2023 and 2024 season from Pfizer, Moderna and Novavax.
Still, Pfizer pulled in $38 billion in revenue of Comirnaty in 2022, though quarterly sales have fallen dramatically since.
Another of Pfizer’s more recent vaccine triumphs was the approval of a respiratory syncytial virus (RSV) vaccine in March. Pfizer was among several pharma giants vying for the market, and Abrysvo’s approval in August followed the FDA’s nod for GSK’s Arexvy just a few months earlier.
Still, there’s tremendous unmet need for RSV vaccines, particularly among older adults and infants — to protect babies from infection, Abrysvo was approved through immunization of pregnant mothers.
Pfizer has one more vaccine in its portfolio: Ticovac, the only shot used to prevent tick-borne encephalitis in the U.S. Ticovac was approved in 2021 to protect people traveling to endemic areas, including parts of Europe and Asia, where the vaccine has been distributed since 1976.
Pfizer’s work in vaccine development doesn’t stop there — the company has more than a dozen programs in clinical trials, including some that make use of the mRNA technology employed for the successful Comirnaty.
In early stages, the pharma giant is using the genetic vaccine platform to develop a combination COVID-19 and influenza vaccine and a shot for chickenpox. And in later stages, Pfizer is working on vaccines to widen the age window that can receive the COVID-19 vaccine, as well as a separate influenza vaccine